Sabrina Grigolo1, Italia Agresta1,2, Stefano Mazzariol1, Dominique van Doorne1
1European Patients’ Academy / EUPATI Non-Profit Organization
2Italian national Association of People with Rheumatological and Rare Diseases (Associazione Nazionale Persone con Malattie Reumatologiche e Rare – APMARR)
Tendenze nuove, Numero Speciale 4 2021; 161-178: DOI: 10.32032/TENDENZENUOVENS04202111.PDF

1. Background
In recent years, a new concept of patient-centred care has emerged. Health systems underpinned by value-based medicine and by a lean organizational model presuppose an effort to ensure “value for the patient”.As a result, a number of questions arise: Where do we situate, and how do we consider, the patient as part of the system? What do we mean by“value”?Are patients involved in defining what is meant by “value”in health, with the implications this will have for policy and decision-making? Is value to be understood only as that of the patient, or also the caregiver? Which operational tools implement patient value? Can diagnostic and therapeutic care pathways (DTCP) be a major tool for patient engagement? What is the current status regarding these points in Italy?
If “patient value” is indeed a major consideration, the initial approach has to be modified accordingly: the focus must be placed not so much on the patient at the centre of the system as on the caring relationship between the various actors, because it is here that value is produced (as confirmed, inter alia, by healthcare litigation data). The patient is just as much a part of the healthcare system as all the other actors, being the main stakeholder in their own health. Alongside the patient, we can find the expert patient (a figure that has emerged in recent years) and the patients’representative. Both these categories collaborate and cooperate with doctors and healthcare providers for continuous enhancement of research, availability and delivery of treatments, thus co-generating the “value” of the healthcare supply chain (Figure 1).
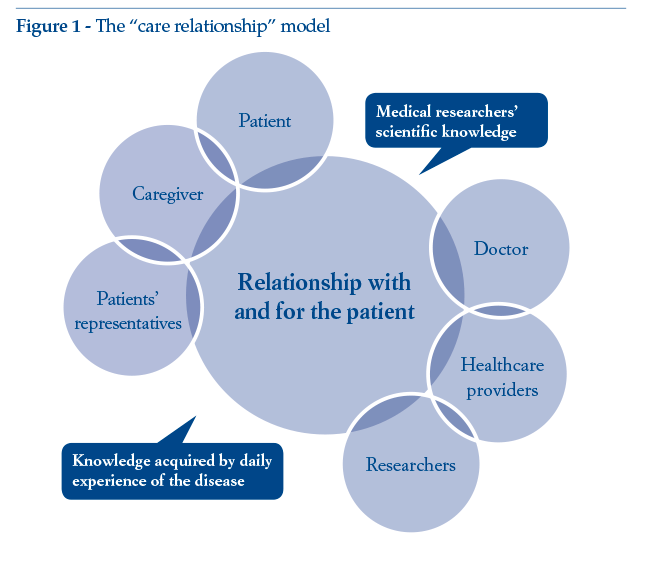
Introducing innovative tools to support the relationship among the various actors in the system is the priority: there is a need for “algorithms”connecting the various components and enabling dialogue among the various parties. The “patient”thus becomes the link between health and illness, between scientific and experiential knowledge. The caregiver acquires experience of the disease alongside their loved one, remaining at their side through thick and thin. In most cases, the caregiver becomes the chief support underpinning the patient’s life and hopes.
The figure of the caregiver (and the expert caregiver) can in some cases actually take on a leading role, as in the case of partially or totally disabling disease that makes the patient unable to take informed, conscious decisions regarding their own treatment pathway.
Thanks to the rise of empowerment and related social movements in recent decades, patients and caregivers are no longer alone, or passively dependent on decisions taken by others: there are now large numbers of patients’associations that, by their collective effort, have enabled identification of new needs, prioritization of new discussions and creation of new opportunities, thus determining pathways for cooperation with institutions. Patients gain in self-assurance and become accredited interlocutors for doctors, researchers, healthcare providers, administrators and public policy-makers. They become a resource for the health system, able not only to advocate for the needs of the patient community they represent, but also to integrate the patient’s perspective into healthcare decisions, to modify healthcare procedures and, last but not least, to play their role in the joint design of their treatment pathway within the broader DTCP roadmap.
In this overall setting of interpersonal relations involving many different parties, the continuum that encompasses information, reception facilities and needs-based care becomes the basis for the relationship of trust between the various actors concerned.
Interpersonal relations can be meant to ensure:
•diagnosis, with the healthcare provider asking the patient the necessary questions. This presupposes that the patient is able to give exhaustive, complete answers;
•concordance between healthcare providers and the patient/caregiver, with a view to optimizing patient care in relation to quality of life and achievement of shared decision-making;
•therapeutic education and adaptation of lifestyle to the patient’s new status: this underlines the disease’s major impact on the patient’s life system, involving as it does their family and social network;
•better quality of life in its terminal phase, with healthcare providers helping to create the best possible care setting in order to reduce suffering and pain.
This means that the relationship between the healthcare provider and the patient/caregiver can have three complementary dimensions:
•cognitive: the healthcare relationship means transfer of information between one component and another;
•technical and gestural: the healthcare relationship enables the patience to learn new techniques, enhancing their know-how;
•emotional-affective-behavioural: this relationship enables the patient and the caregiver to express their difficulties and their emotional experience, making it possible to identify attitudes requiring specific professional intervention with a view to reinforcement and/or remodulation.
This is where it is particularly important that the healthcare provider should, indeed, “take care of”the patient, with the professional’s scientific knowledge offering an opportunity to adapt one’s life to major change, within an educational relationship based on significant learning.
Healthcare requires therapeutic education programmes (in other words, secondary and tertiary prevention), based on methodologies underpinned by brain-based activities consistent with underlying needs. Hebb’s principle, as described in 1949, postulates that significant, repeated, moving experiences create the basis for increasingly stable cognitive structures(1). Research in recent decades has highlighted the human brain’s plasticity throughout its entire lifespan, since it always remains subject to a series of changes determined by what one learns. These changes can be the basis for enhancement of “intelligence”, meaning the capacity to respond effectively and efficiently to given stimuli that have to be interpreted and will then form the basis for decision-making; to trigger action resulting from this; to critically review one’s own interpretations and actions; and to adapt them when necessary. Important functions for significant personal learning include attention, planning, results orientation, behavioural organization, strategic thinking, self-control, self-restraint, self-monitoring, and regulation and control of emotions and motivation(2).
To these components of the learning process can now be added the opportunities provided by technological applications. In this sense, digital therapeutics (DTx) can contribute to the daily life of many patients obliged to “learn”a new lifestyle, adapting their daily rhythms and choices to the illness that has become part of their lives. These treatments can achieve a theoretically optimal condition, in that they can support, correct and jointly create cognitive behavioural capacities essential for effective coping and self-care. A fundamental and peculiar feature of DTx is the patient’s and/or caregiver’s active involvement. This is why, to achieve their full potential, application of these technologies requires a pathway of user profiling and education that must reasonably be part of the clinical practice scenario within which these technologies are implemented.
DTx come into their own in an overall setting within which mobile electronic devices are readily available nationwide. As shown by statistics, these devices are now almost literally part of our clothing and our accessories: we carry them about with us everywhere. What better instrument to enhance patient compliance and monitor individual health, outside an institutional healthcare setting and as part of our day-to-day life?
If the hardware and the algorithms are now available, the main gap is probably the lack of a strategy for the overall system. Germany, England and France have national digital health policies. An article by Sergio Pillon(3)about application of digital medicine in German healthcare practice, as regulated by a law of 1 January 2020, highlights a number of points that can simplify the patient’s and caregiver’s life, such as the following:
•apps are medically prescribed once they have passed safety and operational testing. Their use is thus no longer unregulated, but is synonymous with evidence-based practice ensuring full efficacy and safety;
•telemedicine is becoming the norm. Home and clinic are no longer two worlds totally apart, so that the patient and caregiver are no longer obliged to attend clinic and hospital appointments, other than in an emergency and/or for scheduled interventions (examinations, medical checks, etc.);
•digitalization of a patient’s medical history makes this a virtual record encompassing disease progression, diagnostic tests, medical reports, etc. Above all, this can be an important tool for reconnaissance and pharmacological reconciliation, because to date there is no readily available summary of the individual patient’s different medical prescriptions, other than in cases where patients themselves are meticulous and systematic enough to keep a simple, accessible record of all drugs prescribed and/or taken.
Use of technology can be an asset with a view to promoting patient compliance. Devices dispense treatments and advice, reminding us of when we last took our medicines or have to take them again, showing us how to optimize our lifestyle, displaying our dosage regimen and advising us to consult the doctor when anomalous data are flagged, thus becoming a sort of healthcare watchdog(4). Digital medicine thus marks a cultural sea change in traditional healthcare: more than the simple adoption of new technologies, it allows delivery of services, supply of materials, participation in experience, accessibility and usability of extensive and varied content, as well as new connections between people, places and things(5).
Digital medicine has a place in many diseases, not always necessarily classified as chronic. It can also be an important asset for patients with rare diseases, currently faced with the fundamental challenge of transitional care. This means making the transition from a paediatric to an adult care setting. The advent of digital medicine is thus a change that can determine quality of life, safety of treatment and, last but not least, patient-caregiver-healthcare provider concordance.
Another aspect that should not be underestimated is enhanced quality of life, not only for the patient but also for family and loved ones, particularly bearing in mind the advantages of better patient compliance, reduced need for care and accompaniment, limitation of journey time to/from clinics and hospitals, and reduction in time off work or in work-related constraints for the patient, and possibly also for family members.
2. Examples of DTx in practice
To offer practical examples illustrating who could benefit from DTx, and how, it is appropriate to look at a number of clinical cases differing not only in patient and caregiver needs, but also in the system of healthcare relations. To provide a representative overview, three cases with different diagnoses have been chosen: a child with autism, an adult with stroke sequelae, and an elderly woman with head injury sequelae.
Common to all three cases is the type of information shared between healthcare and social service staff, patients and caregivers. Classified as sensitive health data, this information refers to the subject’s health status; data processing is carried out, in the interest of the subject’s health, by healthcare institutions or medical professionals.
As we will see below, data processed for management of these cases comprise:
• diagnostic data (e.g., diagnostic examinations carried out by a specialist, in a hospital department, at a GP’s/paediatrician’s clinic or pharmacy, or by a physician on house call);
• treatment data: choices of treatment and DTx, with assessment of patient and caregiver compliance;
• rehabilitation protocol data: nowadays, a lot of rehabilitation is done by means of technology at home, with tablets, smartphones and consoles playing an important role in reinforcing messages from professionals such as physiotherapists, speech therapists, etc;
• constant exchange of vital parameter monitoring data. The patient remains at home, while health data are relayed to a telemonitoring station, normally run by hospital staff.
Certain features are common to all three cases presented below:
• presence of a caregiver/family member;
• need to acquire capacities and skills for coping with life change;
• need to feel as autonomous as possible in day-to-day life;
• presence of a contact person/service for treatment;
• responsibility of health services, with activities integrated into DTCP;
• need for a “reconciled”treatment plan (Italian Ministerial Recommendation 17);
• need for close management of daily life, enabling early warning of signs or symptoms related to possible complications;
• individual patients/caregivers to have their own devices.
| 5-year-old child Young parents, Italian father, Peruvian mother. Both very present. Case of autism associated with X-linked genetic disease. Self-sufficient in motor and physical function, is growing well, walks, indeed is hyperactive and moves a great deal. No speech, no verbal expression, only sporadic vocalization. Does not make eye contact with people around him and therefore does not interact with them. Lives in a world of his own. Has no relationship with other children of his age. Relationship with the adult world limited to gestures and touch – e.g., if he is thirsty he goes to the refrigerator, opens it and then puts his hand on a bottle. Incontinent, but feels the need to evacuate; his mother has learned to recognize the accompanying gestures and facial expressions, and takes him to the toilet.A very lively child, with a lovely smile; very fond of music (the father is a musician). Likes water and would happily stay for hours in the bath or in a swimming pool. Very perceptive and sensitive.Enormous healthcare burden: need for round-the-clock supervision for everything. For a year, has been doing psychomotricity, speech therapy and a specific treatment pathway for autistic children, with poor results.Probable need for targeted stimuli and personalized interventions that could at least give him a little more autonomy and, above all, improve his possibility and capacity of communicating with, and relating to, others – both children and adults. |
| Family profile and areas in which DTx are used Luca’s parents (we have given him an imaginary name here) are very attentive to his health condition. They do everything possible for him, striving to create conditions conducive to his receiving the best care. As parents, they have shown their ability to deal continuously with his needs. They have built up an effective relationship with institutions, both for schooling and for healthcare.Luca’s mother does not work and deals full-time with his education. The father is a musician, often away, but very present in terms of contribution to the child’s education. Single income family.Luca has very good digital literacy: he uses the tablet, mobile phone and computer, not only with dedicated programmes, but also with others such as various electronic games. Watching TV calms him down. |
| Application of DTx for Luca• Cognitive behavioural therapy through use of games• Significant learning support • Role of communicator or linguistic mediator. |
| Application of DTx for the parents• Cognitive behavioural therapy for support in day-to-day life• Significant learning support• Constant assessment of the caregiver’s stress level and/or possible burnout• Resources network readily consultable. |
| Giovanni, 48 years Fifteen years ago, had a thromboembolic stroke that left him for about two months in a coma; residual left hemiparesis and slight dysarthria. Unmarried, was very active and cultured (qualified architect), but in cognitive terms has developed a marked memory deficit for recent events, an attention deficit and a slowing down of ideomotor function that has gradually worsened in recent years.On the other hand, the hemiparesis and dysarthria have improved and only slight symptoms remain. This improvement was helped by intensive physiotherapy immediately after the incident.Over the years, the family have consulted a lot of specialists, particularly neurologists, in search of a miracle cure that could bring his memory back and improve his cognitive problems. This has not really proved helpful for him, but has tended to make his interpersonal relations rather hostile and conflictual. Perhaps there is a need for other, specific forms of care to address his limitations. |
| Family profile The parents are very present, but after years of knocking at different doors and undertaking journeys of hope have become resigned. They have consulted large numbers of experts, both medical specialists and healthcare providers, in search of a cure that could “bring back the Giovanni we knew”. He has a sister who, while close to her parents in striving to give active support, has a family of her own that she must be with.The father is retired, while the mother does housework for a family living nearby.The parents are worried about the present and future: • Today: how can they leave the house, even to go to the shops round the corner, and leave Giovanni alone? • Tomorrow: what will happen to Giovanni when they are no longer there? |
| Application of DTx for Giovanni• Cognitive behavioural therapy, through games activity and connections to long-term memory • Significant learning support by application of executive functions. |
| Application of DTx for the caregiver• Significant learning support for addressing a variety of day-to-day needs• Constant assessment of caregiver’s stress level and/or possible burnout• Resources network readily consultable• Monitoring of Giovanni’s movements, health condition and vital parameters (if necessary). |
| Maria, 75 years In a fall from her bicycle six months earlier, she suffered concussion, with subdural haematoma. She resumed walking shortly afterwards and is quite self-sufficient in her day-to-day life, except in some circumstances where she needs help and stimuli. Automatic actions that are simply a question of carrying out movements are not problematic – e.g., going upstairs, getting up, sitting down, eating once she has food in front of her, washing once she has a face sponge and soap ready to hand, etc.For all other situations requiring even limited reasoning, she has difficulties – e.g., sowing on a button, looking for an item of clothing in the right wardrobe and putting it on, tying up her shoelaces, crossing the road, reading a few lines in the paper and repeating what she has just read. |
| She needs almost continuous supervision and assistance. The family is present but needs to be supported, since they underestimate some aspects of her illness and amplify some aspects of her behaviour without managing to help much.She has done courses of rehabilitation, but of a non-specific nature. What would be needed is a personalized pathway to develop and gratify the self-sufficiency she shows in some areas, but at the same time helping her to realise what her current condition is and work to improve it. |
| Family profile Maria has a son and daughter living with their families not far away. She lives with a carer. At least once a day her children visit her. They would be much more involved if they were able to stay connected with her at all times of day. This is not possible, for two reasons: their mother’s functional limitations after the accident; and the cost of the technology and related subscription fees needed to address the mother’s condition and the children’s needs. |
| Application of DTx for Maria • Cognitive behavioural therapy to enhance fine hand movements • Significant learning support by application of executive functions for better motivation and self-esteem • Patient compliance, given the need to take different courses of daily medication (>5 different drugs per day) • Monitoring of vital parameters, given that she has various chronic complaints and is in polytherapy. |
| Application of DTx for caregivers (carer and/or children)• Significant learning support to successfully carry out different daily activities • Constant assessment of the caregiver’s stress level and/or possible burnout • Resources network readily consultable • Remote monitoring of patient compliance and safety of treatments. |
Overall, looking at the clinical application of DTx, the following aspects can be highlighted:
Therapeutic indications (for what):
Which of the patient’s needs are addressed by DTx?
In the R&D process leading to new health products, patients’needs and the amount of access they have to technology are often not the same as for the clinicians and researchers who interpret their data and represent them. Effort should be dedicated to setting up a concrete, real pathway to ensure the patient’s engagement in the R&D phase prior to introduction of new DTx.
Such efforts to enhance patient involvement (inter alia in the setting of recently developed research models such as comparative effectiveness research)(6) could make for more effective research, to include such methods as the following:
• identify patient, caregiver and community priorities;
• collect feedback on the relevance and urgency of the priorities thus identified;
• test the usefulness and relevance of the questions asked, and their applicability to the real-world scenario;
• identify outcomes of interest;
• perform a reality check;
• identify the best approaches for selecting and collecting data of interest;
• leverage the motivation generated by participation in an innovation development programme;
• facilitate the involvement of other patients, inter aliaby means of patient associations.
Doctor’s and/or healthcare provider’s prescription (who prescribes)
Training of doctors and healthcare providers is currently a priority. Whoever prescribes DTx must have specific digital literacy skills and clinical experience, enabling them to promote patients’use of these technologies and raise awareness on the subject. For some diseases like attention deficit hyperactivity disorder (ADHD), the prescribers of DTx are speech and/or rehabilitation therapists.
There are three fundamental points related to prescription of DTx:
• clinical efficacy and safety;
• data protection and cyber security issues;
• reimbursement policy.
For the patient and/or caregiver (for whom)
DTx can be used both by patients and by caregivers, albeit with different aims, the ultimate goal in both cases being to achieve a better condition of life at a time of major change in terms of everyday self-sufficiency. Patients can benefit from improved compliance with the treatment regimen and prevention of complications, while also finding a source of support to motivation, attention and concentration. For caregivers, DTx can be very useful with a view to countering emotional fatigue and managing critical issues, as well as in terms of ready access to services and remote monitoring of the patient.
It is particularly important to take into account and thoroughly assess the patient’s and caregiver’s health literacy and digital skills before prescribing DTx. What can happen to a person who is prescribed DTx if s/he does not understand what type of content these applications handle, or does not have basic digital literacy? Another non-negligible aspect is the setting in which DTx will be used, with regard to facilities in the building (connectivity, Wi-Fi, etc.) and availability of personal devices.
Reconnaissance and reconciliation of DTx (what therapeutic implications)
Just as for pharmacological treatment, DTx too should find a place among the tools that enable sharing of therapies between healthcare providers, patients and caregivers, in line with the indications of Italian Ministerial Recommendation 17 of 2014(7).
Reconnaissance comprises collection of complete and accurate information on the patient and on the medicines s/he is taking, this information being indispensable for purposes of correct prescription. Reconciliation of pharmacological treatment is a formal process that allows clear and thorough identification and understanding of the pharmacological treatment taken to date, together with other information on the patient; this allows the prescribing doctor to carefully assess whether ongoing treatment should be continued, modified, partly discontinued or stopped altogether. Implementing these courses of action helps to promote effective personalization of treatment, optimizing its adaptation to the individual patient’s history and needs.
Personalization of DTx raises the need to demonstrate the treatment’s efficacy and its consistency with the single patient’s needs, once the prescribing doctor or healthcare provider has implemented the treatment. This makes it important to identify specific outcome measures, alongside more general treatment endpoints, thus demonstrating the appropriateness of the personalized regimen and any need for modification.
Informed patients: what we know, what we want to know, and how we want to know it (HOW)
The principle that “time spent in communication between doctor and patient is part of treatment time”, unfortunately conspicuous by the absence of its acknowledgment in the current organizational setting of healthcare, is the basis of an effective relationship between the healthcare provider, patient and caregiver (if present). This dynamic includes not only the doctor, but all the figures actively involved in the overall process from pre- to post-treatment: prevention, diagnosis, treatment, rehabilitation and possibly palliative care. Communicating with the patient to convey information so as to obtain their informed consent is thus treatment time.
The patient must be able to give informed consent to treatment, on the basis of information made available by the doctor/healthcare provider that explains the modalities, characteristics and consequences of the intervention. This makes it necessary to adapt language, inter aliaby identifying appropriate tools and strategies that make it possible to achieve an acceptable level of awareness. This is especially true of innovative treatments that differ from routine approaches, necessarily involving appliances or devices designed for other uses (e.g., mobile phone, television, tablet, etc.). The patient must be able to understand and accept all the hypothetical complications of the treatment s/he is about to undergo, and this immediately brings into play the right to be correctly informed.
Another sensitive aspect, on which it is appropriate that the patient should be correctly informed, is consent to data processing. In cases like DTx, the data identified are transferred electronically and this makes it even more important to reassure the patient that information will not be used for purposes other than those s/he has authorized.
The main questions to which patients generally want an answer at the time of informed consent to data processing tend to be the following:
• What do you want to know about me?
• How is the information about me used?
• In what format are these data managed (anonymous or identifiable)?
• How, and where, are the data stored?
• Where will you collect the data from: clinical file, questionnaires, exams, tests, devices, etc.?
• Are we sure that decisions taken with regard to my health/illness are based on my data, not somebody else’s?
• What happens if a hacker accesses the database where information about me is stored?
• Who guarantees that my data are not being used to “profile”my health/illness status – in other words, that they won’t be used by third parties for marketing purposes?
Availability of large quantities of data can be an important factor for the health system, favouring predictable treatment outcomes and the development of increasingly personalized therapies, etc. Given the potential value to society of the health data now available, there is also a rationale for taking the lead in promoting greater empowerment for citizens and patients with a view to sharing such data – obviously subject to appropriate data protection measures.
3. The EUPATI Expert Patient and the R&D process in relation to new (digital) therapeutics
DTx make up a particularly important subset in the overall context of digital health and digital medicine. Details of their regulatory classification, development and availability for the healthcare system are described extensively in other parts of this booklet.
Availability of DTx able to bring real benefit to patients and health systems, with appropriate guarantees to all stakeholders (patients, healthcare providers, decision-makers, manufacturing companies), presupposes attentive management of the R&D process as the leading priority. From this point of view, an important question is: Who are the experts on the disease? Alongside the clinicians and researchers, patients have relevant expertise and skill because they live with the illness, and caregivers share that experience on a daily basis. Since this experiential knowledge has the same level of dignity as scientific knowledge, it is of fundamental importance to research, diagnosis and treatment. Patients and caregivers have needs that clinicians often do not know about, or whose degree of priority they do not understand from the viewpoint of the person living on a daily basis with the specific condition concerned. For this reason, in adopting a patient-centred model, patients’thinking must be factored into decision-making about their health.
EUPATI (European Patients’ Academy on Therapeutic Innovation) is an innovative European project, indeed the only one of its kind, launched in 2012 thanks to input from IMI (Innovative Medicines Initiative). EUPATI involves a consortium of 33 organizations, including patient associations, non-profit organizations, universities and pharmaceutical companies, and is guided by the patients themselves (European Patients Forum). In addition to creating a high-level course for patients on pharmaceutical research and development, which has been successfully completed to date by over 150 Expert Patients from 31 different European countries, the EUPATI project has also developed the online platform “Toolbox”. Available in nine different languages, this major platform contains informative materials explaining the A to Z of pharmaceutical R&D. In recent years, EUPATI has become a certified brand and a synonym for quality in patient training; its input has driven public debate on patient involvement in pharmaceutical R&D. The new Italian offshoot Accademia del Paziente Esperto EUPATI onlusrecently concluded its first training course, graduating 50 Italian expert patients who will be working together with Italian EUPATI Expert Patients trained at European level.
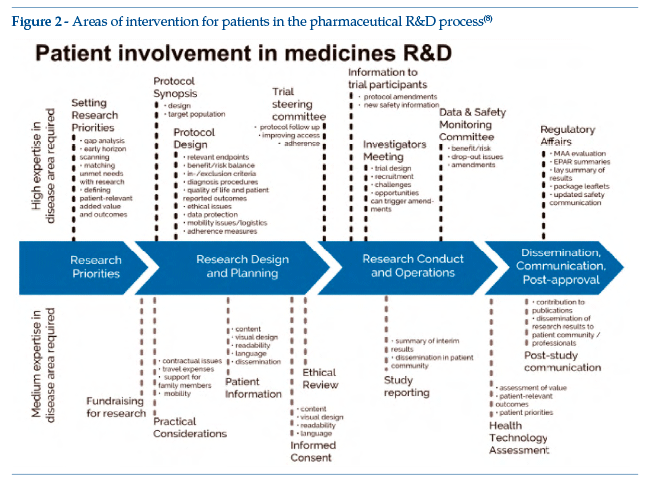
Figure 2 shows the core curriculum of the EUPATI Expert Patient course, on the basis of which training is devised and dispensed by means of classroom lessons, distance learning modules, webinars and forums (at basic and advanced level).
Despite the specificities of these technologies (extensively discussed in other chapters of this booklet), R&D for DTx has many features in common with validation of new pharmaceuticals, including the potential contribution of Expert Patients. Areas of interest here include a number of practical concerns that are fundamental to the patient and caregiver. FAQ in this respect, in addition to those on management of personal data, include the following:
• What does clinical investigation of DTx set out to do?
• Who promotes the investigation?
• Who is my clinical contact person?
• How long does the study last?
• What happens at the end?
• What are the side effects in this specific case?
• What do “screening”and“follow-up”mean in the case of DTx?
• What are the risks and benefits? Are these “safe”treatments?
• What impact can DTx have on quality of life, in relation to the proposed indication?
• Can I withdraw my consent? What happens if I no longer want to take part in the experimental study? What consequences does that have?
• Must I bear any costs (travel, diagnostic tests, drugs, subscription fees, rental deposits, etc.)?
It is in this setting that the Expert Patient takes on an important role: design of a new algorithm must address patients’and caregivers’needs, in the same way as for new molecules. Just as drug R&D involves selection of the most appropriate and user-friendly pharmaceutical forms, for DTx too a system of patient and caregiver engagement is needed in order to set up an interface as closely tailored as possible to the characteristics and needs of the target patients/caregivers.
In an optimal scenario, just as occurs for clinical investigation of drugs, patients should be involved from the very earliest stages of designing the related technology – which means not waiting, as often happens, for the start of pilot clinical testing or usability testing. Asking two or three non-specialists for their comments on the algorithm is not enough. It is also methodologically wrong, just as it is wrong to use a convenience sample for testing an algorithm’s usability. Early, structured involvement of patients and caregivers in the R&D process probably enables maximization of the technology’s potential efficacy and safety, allowing more appropriate assessment of its characteristics and helping ensure that therapeutic performance will address the patient’s most pressing needs.
Looking specifically at DTx, compliance with the criteria and methodological rules of research, with a view to ensuring valid clinical investigation, must be complemented by a number of other considerations that cannot be underestimated at the planning stage:
1. The patient’s and caregiver’s digital skills: not everyone is a digital native. While age can be a factor in this regard, it is important to be systematic in assessing the digital literacy of the person who will be using the tools (and this also applies to healthcare providers);
2.Availability of devices: just as it is important not to take for granted that all patients and caregivers will have the latest model of smartphone or tablet, it should also be remembered that not everyone will even have one;
3.Internet access: not everyone will have taken out a subscription with a network provider, and this is a point that needs to be thought through. How right is it to “advise”people that they should purchase a subscription with an internet provider? In addition, cover can be patchy in some parts of the country;
4.Deposit for rental of devices: this can be a big outlay for some families. When an app is prescribed and the related hardware is available on a loan for use agreement (the most common arrangement), who has to meet the bill for the deposit fee, or for any repairs and maintenance?
In conclusion, development of DTx applicable to many chronic and other diseases, both widespread and rare, is certainly to be seen as an opportunity for health systems and citizens. In these therapies, the patient’s active participation is particularly important – it is, indeed, an essential factor with a view to identifying possible needs and priorities to be addressed by these technologies, and a sine qua nonfor their success. In this respect, it is surely reasonable to propose that patients and/or those who care for them at family/society level should be involved as early as possible in the design and validation of these products. A significant role to this end can be played by figures like Expert Patients, meaning people who have received appropriate and rigorous training that enables them to give effective support in R&D decision-making for healthcare products. In the same way, the importance of the patient’s active participation in the treatment pathway presupposes that prescribing DTx should go hand-in-hand with close prior assessment of expected patient compliance, based on effective and informed use of technology.
What is known
• Our point of view as patients and/or caregivers in relation to treatments is generally different from that of healthcare providers
• Our experiential knowledge, enhanced by specific learning that we acquire autonomously or with the help of experts, is integrated with the scientific knowledge of healthcare providers
• Knowledge of our history as patients and/or caregivers is required by healthcare providers, with a view to implementing the best possible personalized treatment pathway.
What is uncertain
• The rationale for the healthcare provider’s decision-making (concept of shared decision-making).
What we recommend
• Shared decision-making based on healthcare provider-patient concordance, with the caregiver’s support
• Giving due recognition to the experiential knowledge of the patient as a fully fledged partner in the treatment pathway, including the R&D of new treatment products
• Clear clinical indications for DTx, prescribed by healthcare providers with the necessary expertise and skills
• Integration of DTx into therapeutic reconnaissance and reconciliation processes (e.g., Italian Ministry of Health, Recommendation 17 – 2014)
• Basic digital skills for the healthcare provider
• Patient’s and caregiver’s level of confidence with DTx to be ascertained.
Bibliography
1.Hebb DO. The organization of behavior: a neuropsychological theory. Wiley, New York, 1949.
2.Trinchero R. Per una didattica brain-based: costruire la learning readiness attraverso la pratica deliberata. Form@re 2015; 3: 52-66.
3.Pillon S. https://www.agendadigitale.eu/sanita/sanita-digitale-la-nuova-legge-tedesca-e-il-vuoto-legislativo-delitalia/
4.Recchia G. Salute Digitale, la corsa è giàiniziata.Tendenze Nuove 2019; 2: 3-6.
5.Meskó B, Drobni Z, BényeiÉ, et al. Digital health is a cultural transformation of traditional healthcare. MHealth 2017; 3: 38.
6.Mullins CD, Abdulhalim AM, Lavallee DC. Continuous patient engagement in Comparative Effectiveness Research. JAMA 2012; 307: 1587-8.
7.Raccomandazione n. 17 – Riconciliazione della terapia farmacologica, a cura di Ministero della Salute – D.G. Programmazione sanitaria, dicembre 2014.
8.Geissler J, Ryll B, di Priolo SL, Uhlenhopp M. Improving patient involvement in medicines research and development. Ther Innov Regul Sci 2017; 51: 612-9.



