Elisabetta Ravot1, Roberto Ascione1
1Healthware Group, Milano-Salerno
Tendenze nuove, Numero Speciale 4 2021; 105-116: DOI: 10.32032/TENDENZENUOVENS04202106.PDF

The development of a business model and access policy for digital therapeutics (DTx) is becoming a recognized need and a matter of discussion in many countries, which can benefit from examples already in place in other systems. Until recently (last update of this article: April 2021), forms of reimbursement policy for digital health solutions, including DTx, were mostly lacking in many parts of the world; this scenario is nevertheless quickly changing, having received considerable impetus from recent developments in a number of countries. Of particular interest in Europe is the recent approval of the Digital Healthcare Act (DVG, Digitale-Versorgung-Gesetz) in Germany, introducing the possibility of an accelerated pathway to the market for DTx. Taking such developments into account, the aim of this paper is to present a brief overview of the most representative features internationally, with the focus on a number of key countries.
In the USA, the lack of free medical care for all means that the onus for payment of digital healthcare solutions is to a very large extent borne directly by users/patients. This creates a patchwork scenario, with examples of digital health or DTx solutions paid directly by users, alongside growing numbers of cases where the costs are covered by a number of different players. Some digital health solutions have acquired great popularity – e.g., OneDrop (diabetes management), Headspace and Calm (mental health/meditation); such products attract millions of paying users, normally with a monthly subscription fee credited directly to the developers. In addition, private health insurance schemes and a number of hospitals are starting to develop or to license out their own digital health solutions, in order to reduce the typical user’s risk profile (in the case of insurance companies), or to provide more adequately for the population’s specific health needs in the catchment areas concerned, while also (in the case of hospitals) promoting achievement of federal targets for reduction of hospital admissions. Another emerging form of reimbursement in the United States is the inclusion of digital health solutions in the healthcare packages of large corporations, which subsidize treatment costs by including these solutions in corporate employee benefit packages.
Promoting access to digital health approaches and tools is an important objective in the USA’s mid-term planning, a case in point being the inclusion of DTx in the 2020-2025 Federal Health IT Strategic Plan of the Office of the National Coordinator for Health Information Technology. Among the strategies identified, the plan aims to develop evidence-based use of DTx as a treatment option for prevention, management and treatment, by use of smartphones, tablets and other personal devices.
In addition, as a result of the current CoViD-19 pandemic the FDA has further stepped up its active support for the approval of prescription DTx. Evolving the pre-existing “Pre-Cert”programme, designed to regulate and accelerate the development of software as medical devices (SaMD), the FDA issued guidance documents introducing appropriate degrees of flexibility on a large number of regulatory requirements, including a temporary waiver of premarket notification requirements under section 510(k) of the Food, Drug, and Cosmetic Act. In January 2021, these relaxations of previous regulatory requirements were made permanent for 91 devices and SaMD tools, out of the 221 Class I and Class II medical device types covered by the waiver categories. Details of the updated waiver status for the classes concerned are available at:
https://www.federalregister.gov/documents/2021/01/15/2021-00787/making-permanent-regulatory-flexibilities-provided-during-the-
CoViD-19-public-health-emergency-by#p-64 (last accessed March 29, 2021).
In the United Kingdom, the National Health System (NHS) has for some time offered a broad catalogue of digital solutions dedicated to health and wellbeing; these are certified and accessible via the NHS app library, which is in turn part of the NHS app. In some cases, the NHS has negotiated licensing agreements with digital health app developers, in order to make these solutions available free of charge to all patients. The apps falling under this heading are mostly intended to support the user/patient in playing a more active role towards the management of their mental and physical health. To be included in this catalogue, products have to demonstrate compliance with the standards defined by NHS Digital,including the need for evidence of clinical outcomes achieved or reported by patients, clinical safety and technical stability; information is also required on data protection, usability, acceptability and interoperability. Details of assessment criteria used by the NHS for health apps, and related digital tools, can be found via the following link:
https://www.nhsx.nhs.uk/key-tools-and-info/digital-technology-assessment-criteria-dtac/ (last accessed March 29, 2021).
The NHS roadmap for patient digital services is broad, diversified and still evolving. The “Empower the Person Roadmap”, which is periodically updated, provides information on the status of resources (available, recently introduced, under development, expected). The complete roadmap,now in its fifth version, is available at:
https://eu-rm.roadmunk.com/publish/0dbd8ebaa98e26573e09b1d1fc74433d486ee5d2/ (last accessed March 29, 2021).
It is also interesting to note that the NHS has complemented this promotion of digital services by setting up the NHS Digital Academy. A joint venture between the NHS, Imperial College London, the University of Edinburgh and Harvard Medical School, the Digital Academy aims to promote training and development for a new generation of digital leaders, able to guide the transformation of the NHS.
At European level, in April 2019 the European Commission (EC) published its “Proposed Guiding Principles for Reimbursement of Digital Health Products and Solutions”as one of four working papers developed collaboratively by members of the eHealth Stakeholder Group (eHSG), the EC’s expert group on European digital health policy. The paper contains recommendations that address the peculiar nature of digital health solutions and their difficulty in entering funding and reimbursement pathways in Europe, namely:
• specific criteria are needed to make appropriate reimbursement decisions for digital health products and solutions;
• relevant digital health products and solutions should benefit from either EU or national funds within innovation investment funds;
• European guidelines for relevant and fit-for-purpose evidence generation for digital health products and solutions should be developed;
• the specifics of digital health products and solutions must be considered when developing instruments for assessing and rewarding the value they provide for patients, healthcare actors, health systems’ sustainability and society.
The paper can be downloaded at: https://www.medtecheurope.org/wp-content/uploads/2019/04/30042019_eHSGSubGroupReimbursement.pdf (last accessed April 7, 2021).
In France, a significant example of reimbursement for a digital health platform is that of Diabeo (Voluntis), a digital medical device for insulin dosage. The HAS (Haute Autoritéde Santé) has approved reimbursement of Diabeo, solely for cases in which it is prescribed in the context of a telemedical consultation; the background to this decision is the 2019 authorization for doctors in France to offer teleconsultation, via approved telemedicine platforms, as a recognized activity for which they are paid.
The case of Diabeo in France can obviously create a precedent that can facilitate and speed up comparable approval measures for reimbursement of other digital health solutions, including DTx. It is reasonable to think that the dynamics of access to reimbursement in a teleconsultation setting will bring forward the development of further solutions by providers interested in accessing the French market (or that this precedent will provide a rationale for developing digital health or DTx with a view to their being prescribable and usable on a teleconsultation basis).
A further example also deserves mention in France: the HAS has included in its reimbursable list for health products and services Moovcare Poumon, a medical device based on software for telemonitoring of relapsesand complications during follow-up of lung cancer patients at high risk of relapse. The original document containing the HAS approval can be accessed via the following links:
(last accessed March 29, 2021).
In Belgium, at the initiative of the federal government in collaboration with multiple stakeholders, the mHealthBelgium platform is available for mobile applications with CE marking as medical devices. This platform centralizes all relevant, necessary and valid data for any such applications to be used by patients, healthcare professionals and healthcare institutions.
The platform (accessible in Dutch, French and English) provides information on CE marking, the GDPR, conformity, compliance with safety and authentication rules, as well as the cost and financing of the app concerned.
Apps on the platform are classified on the basis of a 3-tier validation pyramid:
• M1, the basic level, includes criteria such as CE certification as a medical device and GDPR compliance;
• M2 is based on criteria regarding interoperability and connectivity to the eHealth platform;
• M3 is for apps that can demonstrate added value in socio-economic terms and are eligible for reimbursement.
Overall management of mHealthBelgium is carried out by beMedTech (the medical technology industry federation) and Agoria (the association representing companies in the technological sector), in close collaboration with three national authorities:
• the Federal Agency for Medicine and Health Products (FAMHP), as the authority responsible for safety, quality and efficacy of drugs and health products, which is responsible for tier M1;
• the eHealth platform, as the federal eHealth organization implementing the infrastructure for exchange of information in the health sector, which is responsible for tier M2;
• NIHDI, the National Institute for Health Insurance and Invalidity, responsible for reimbursement of health products and services, which is responsible for tier M3.
Further details can be found at the following site:
https://mhealthbelgium.be (last accessed March 29, 2021).
The most relevant development in Europe is the approval by the German Parliament of the new rules supporting digital innovation in Germany. The new roadmap dates from December 2019, with the introduction of the DVG (Digitale-Versorgung-Gesetz), a specific regulatory framework for digital health applications (DiGA, Digitale Gesundheitsanwendungen), which entered into force on 21 April 2020. The Federal Ministry of Health order setting out implementation of the DVG can be consulted online (https://hih-2025.de/wp-content/uploads/2020/04/DiGAV_RefE.pdf; last access March 29, 2021). On 27 May 2020, the BfArM (Bundesinstitut für Arzneimittel und Medizinprodukte) started to receive the first applications; the starting date for the register, with the inclusion of the first DiGA, was scheduled for October 2020 (Figure 1). It was announced recently that the DVG will be iterative in nature: consistent with agile developmentprocesses, the regulations will follow a sequence of learning and adaptation over a period of time.
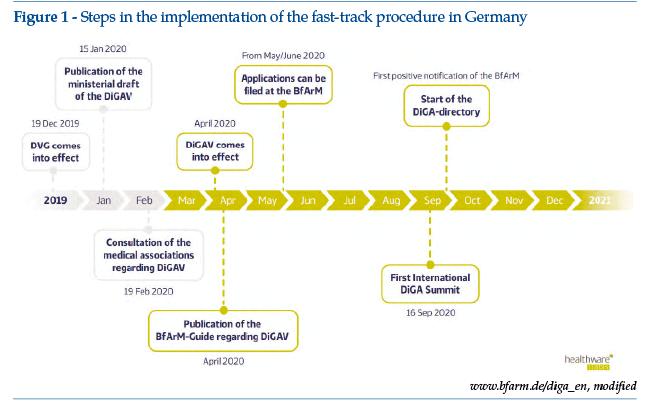
The new German regulations, potentially impacting 73 million citizens covered by the national health service and their GPs, aims as a whole to harmonize developers’needs (rapid placement on the market and minimization of obsolescence risk) and the regulatory requirements for medical devices, in relation to the need for scientific validation before placement on the market.
The cornerstone of the regulations is the setting up of a fast-track approval process for digital health solutions (see Figure 2).
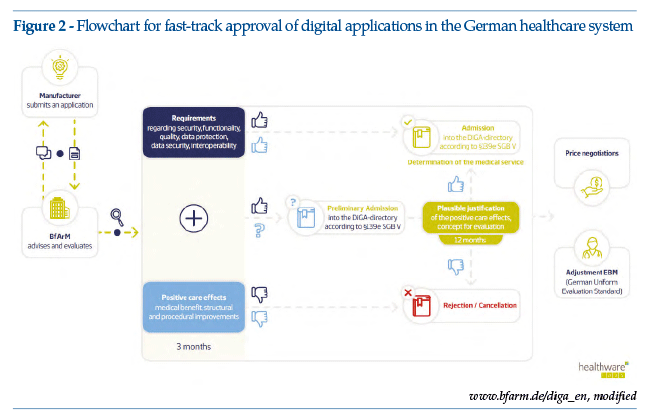
As of May 2020, less than a year after the first draft of the DVG was drawn up, health app suppliers started to make fast-track application to the German regulatory agency BfArM, supported by documentation of the scientific development process together with technical information on data safety and on the app’s operational testing/results. BfArM has issued specific guidelines on fast-track applications, for manufacturers, service suppliers and users. The guidelines are probably the most complete and accessible document regarding the regulations and their application; an English version, published at the end of August 2020, is available online (https://www.bfarm.de/SharedDocs/Downloads/EN/MedicalDevices/DiGA_Guide.pdf?__blob=publicationFile&v=2; last accessed March 29, 2021).
On 16 September 2020, the first International DiGA Summit took place. Examining the regulatory state of the art, the event was attended by over 1,500 participants from more than 40 countries, with contributions from BfArM and the Federal Ministry of Health.
At the present date (April 2021), twelve DiGA are included in the DiGA directory (https://diga.bfarm.de/de; last accessed April 9, 2021).
The main points in the current German scenario can be summed up as follows:
• DiGA are defined as CE marked medical devices with a low risk classification (classes 1 and 2a), whose main function is based on digital technologies. Designed for health treatment or as a support for identification, monitoring, treatment or mitigation of a disease or disability, they can be used by the patient independently, or with the help of a healthcare professional;
• DiGA can be prescribed and reimbursed only after approval by the BfArM and inclusion in the DiGA register;
• The DiGA must have demonstrated one or more positive effects on health. These effects can comprise a medical benefit, or a relevant improvement for the patient in terms of treatment content and processes. Examples of the latter category include patient compliance, autonomy and health literacy, together with facilitation of healthcare access and further outcomes related to improvement of the patient’s everyday life;
• Studies to generate evidence of benefit must be carried out in Germany. In specific cases, studies carried out wholly or in part in other countries can be accepted, on condition that healthcare status and the study population are similar to those in Germany;
• The supplier can apply for definitive or provisional registration. In the event of provisional registration, a test period of up to 12 months is granted; it should be borne in mind that the supplier can apply only once for provisional registration. If an application for definitive registration is rejected, the supplier can no longer obtain provisional registration, but must wait a year before presenting a new application (obviously supportedby further data and evidence, in addition to those presented at the time of the first application). The supplier must therefore carefully weigh up the pros and cons of applying for definitive or provisional approval, on the basis of already available and pending evidence. The BfArM provides informal consultancy on these matters;
• DiGA suppliers can demonstrate the efficacy of their solutions on the market within one year, and negotiate prices with the umbrella organization for public health insurance cover in Germany (GKV-SV, Gesetzlichen Krankenversicherung Spitzenverband);
• Doctors are authorized to prescribe health apps that have passed the required data safety and operational tests, and have been placed on the BfArM register (Figure 3).
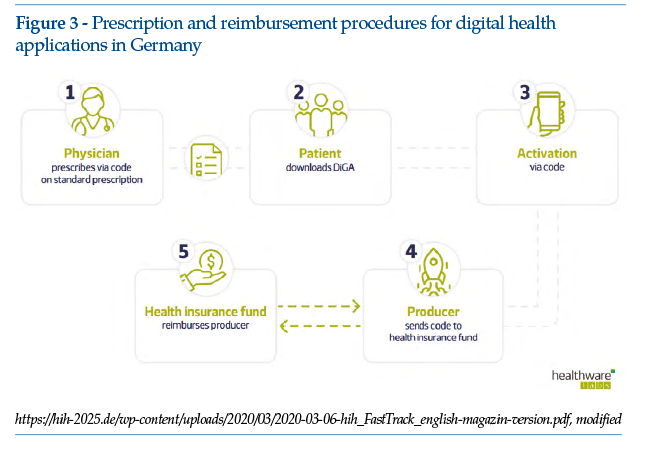
• Telematic consultations are becoming the norm. Healthcare professionals must actively promote telemedicine services to patients, for example on their websites. Prices for telecare services are still under negotiation, but should soon be defined;
• German public healthcare insurance bodies must send details of patient demographics and health status in anonymous form to a central databank managed by the German government. Research organizations and universities can request access to data for research purposes. This will guarantee better access to patients’data for research;
• Every insured person will have access to an electronic medical file. By January 2021, this access must be available to every person covered by a public health insurance scheme (73 million people);
• Doctor-patient communication and prescriptions, initially in hard copy, will gradually be migrated to electronic channels. Electronic prescriptions for medicines, treatment and laboratory/instrumental examination, specific indications by the doctor and certificates of invalidity for employers will be allowed. Traditional communication by fax will not be encouraged;
• Pharmacies, healthcare professionals and hospitals will be required to use secure data networks. All pharmacies, hospitals and healthcare professionals must connect to the German network for secure health data, made available by Gematik. In the event of non-compliance, they will be liable to fines;
• Health insurance bodies will make online enrolment available to those covered;
• This innovation of health services will be subsidized: the German government has made annual funding of €200 million available for another four years, in support of innovative projects in the health sector.
Illustrative cases of reimbursement policy for digital health or DTx
Globally, an increasing number of digital health or DTx products are being considered for coverage by local, regional or national payers. TheCoViD-19 pandemic has in fact exposed the challenges payors face in deliveringaccessible high-quality care outside of traditional office-based visits. Payors are now considering additional options for how to best meet patient needs and close gaps in care with non-traditional approaches. While the list of reimbursed products is evolving, some“historical”examples still provide reference cases.
A significant example of accessibility, involving the UK and US markets, is that of Sleepio, a customized DTx product for management of sleep disturbance, which is based on cognitive behavioural therapy (CBT) techniques and has been clinically validated (33 peer-reviewed scientific publications, including eight randomized controlled trials – RCT). On the basis of the clinical evidence obtained, the American College of Physicians has recognized Sleepio as the first line CBT in treatment of chronic insomnia. Thanks to a partnership with the NHS, Sleepio is now freely available in a number of UK regions (including 8 million users in the London metropolitan area and 2.3 million in the Thames Valley). In the US, healthcare benefits management company CVS has included Sleepio in its formulary and over 2 million users are privately covered by employeers that include this app in employee benefit plans; currently, over 12 million people thus have free access to Sleepio.
Conclusions
The DTx industry is still in its infancy and rapidly evolving and, whileclose attention is rightly given to setting up regulatory and clinical development roadmaps, it is also important to consider that the generation of significant revenue flows is, and will remain, one of the main challenges for this sector on all markets.
Europe is no exception in this respect, having opened the road towards a serviceable access model; the recently introduced German regulations, which mark a definite step forward along this road and can be seen as a strong catalyst, offer a model that other European countries can take inspiration from.
Currently, DTx are not available in Italy, however different players are active in research and development. Moreover, the Italian Medicines Agency (AIFA) has taken the first steps towards regulating DTx in Italy and has made the new objective official in its 2020-2022 Performance Plan, which includes “Proactively monitoring the digital therapy applications currently in the development, and through interaction with the various stakeholders both at national and European level in the form of targeted meetings and workshops, identifying the aspects that fall within the competence of AIFA, acting as a promoter at European level of the process of adaptation of the regulatory system”. Further details can be found at:https://performance.gov.it/performance/piani-performance/documento/1332(last accessed April 13, 2021).
As an Italian Working Group, we are looking with great interest at the evolving scenario in Europe in terms of access and reimbursement policy, and believe there is an urgent need to evaluate and define models that can be practicable and sustainable.
What is known
• In most regions and countries, the lack of adequate reimbursement pathways is among the most important barriers to the digital transformation of health and care. However, actual examples of reimbursement frameworks are increasingly available and some countries already have functioning processes in place. In this overall picture, the CoViD-19 pandemic is acting as a catalyser and it is possible that many contingency measures will become permanent, thus speeding up progress towards access to DTx.
What is uncertain
• One year on from the activation of the DiGA repository in Germany, preliminary and encouraging data on this program are available. A longer period is needed to understand how suppliers are responding, the approval/rejection rate and conversion rate from provisional to permanent approval, the lead time for dealing with applications, etc. The status of these and other indices will shed important light on the practicability and sustainability of this model
• It is not known what the position of the European institutions will be, whether there will be an evolution towards European guidelines, or whether it will be possible for DTx to be centrally approved in the same way as pharmaceuticals by the EMA. Similarly, the role played by regulatory institutions in the individual countries will have to be clarified (e.g., whether in Italy the role currently played in Germany by the BfArM can / must be fulfilled by the Ministry of Health alone, or in cooperation with AIFA and/or other institutions).
What we recommend
• We recommend considering the German case as a basis for developing models in other European countries and, in particular, for outlining an Italian proposal.



