Gianluca Trifirò1,2, Salvatore Crisafulli2,3, Gabriele Puglisi3, Giorgio Racagni2,4, Luca Pani2,5,6,7
1Department of Diagnostics and Public Health, University of Verona
2Italian Society of Pharmacology (Società Italiana di Farmacologia – SIF)
3Department of Biomedical Sciences, Dentistry and Morphological and Functional Imaging, University of Messina
4Department of Pharmacological and Biomolecular Sciences, University of Milano
5Department of Biomedical & Metabolical Sciences and Neurosciences,
University of ModenaNO and Reggio Emilia
6Department of Psychiatry and Behavioral Sciences, Leonard M. Miller School of Medicine – University of Miami
7VeraSci, Durham, USA
Tendenze nuove, Numero Speciale 4 2021; 149-160: DOI: 10.32032/TENDENZENUOVENS04202110.PDF

1. Digitalization in healthcare
Over the past ten years or so, the digital revolution has radically transformed all sectors of society and, more recently, is starting to produce an impact mainly in the healthcare sector. Two fundamental factors are responsible for this trend, which is nothing short of a revolution: i) the quantity of health data generated by each individual patient; and ii) increased computing capacity, in terms both of storage and of analysis. Against this background, not only are the time and space coordinates of the very concepts of health and disease undergoing a profound transformation, but there are also enormous changes to patients’behaviour and to diagnosis and treatment pathways(1). According to the Observatory for Digital Innovation in Healthcare, Italy showed a 7% increase in turnover for digital procedures in the health field in 2018, bringing the annual total to almost €1.4 billion(2).
By the term “digital health”, or eHealth, is meant the use of various digital technologies to support and deliver health services for improvement of individuals’health and well-being, often involving people who are not actually ill. The broad category of digital health includes many different technologies, among them telemedicine, social media, applications (apps), wearable devices, smartphones and digital therapeutics (DTx). These technologies present new opportunities for addressing challenges that are on the rise worldwide, the aim being to improve the quality of health services by putting in place a system of patient-centred care(3). This sector has not yet acquired its own standard vocabulary, but there are three macro-definitions into which the technologies used in the health sector can be bracketed: digital health, digital medicine and DTx. The generaldefinition of digital health includes technologies such as apps or web apps that support users in changing their lifestyle and pursuing goals related to their well-being, generating health data that can, in turn, support research activities and clinical practice. Digital medicine, on the other hand, encompasses a category of clinical evidence-based digital technologies, able to carry out interventions in favour of human health. Finally, the DTx category comprises all those digital technologies that offer evidence-based therapeutic interventions in order to prevent, manage or treat a medical problem or disease(3).
2. The role of digitalization in pharmacological treatment
Most technological applications used by patients are digital versions of so-called Patient Support Programmes (PSP). These tend to be related to organizational or other needs, aiming to give patients support so that they can better manage their illness, understand their overall condition and/or receive counselling on their illness trajectory. Digital PSP are mainly personalized apps, used to promote patient compliance, enhance communication between patients and healthcare providers, offer tools for disease monitoring and, more generally, promote patients’engagement in their own day-to-day healthcare. Considering their capacity to collect data by means of clinical diaries and/or questionnaires, PSP can also be useful for research, obviously in compliance with data protection measures. As applications without therapeutic activity, they are only rarely subject to experimental clinical studies(3).
Unlike PSP, digital medicines are pharmaceutical products in which the drug to be taken is integrated by activation of a sensor after swallowing, the ultimate aim being to guarantee patient compliance. Pharmaceutical nanotechnologies are no longer surrounded by an aura of mystery, and have entered the market for swallowable treatments. These tiny transmitter sensors can be formulated within a capsule or during manufacture of a tablet; once they reach the stomach, thanks to its acid pH, they become activated and transmit information about the digestive process to which they are exposed, thus affording insight into digestive tract function and absorption of the active principle with which they are associated. This would be confined to the realms of mere technological curiosity were it not for the fact that, in late 2017, the Food and Drug Administration (FDA) approved the first product combined with this type of sensor(4). Known by its brand name of Abilify MyCite, the product was developed by the Japanese pharmaceutical company Otsuka (manufacturers of the antipsychotic aripiprazole, or ®Abilify) in collaboration with Proteus Digital Health. With the patient’s consent, once the sensor has been swallowed it communicates with a stick-on wearable receiver similar to a band-aid; from there, information is then passed on to the patient’s (or somebody else’s) smartphone or tablet. The final aim is to measure patient compliance in adults with primary psychoses such as schizophrenia, manic episodes or mixed episodes in bipolar type 1 affective syndromes (Figure 1).
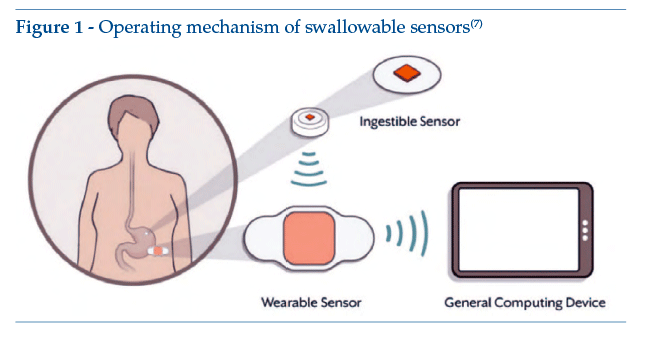
However, this technology has not proved very popular, resulting in major revenue losses for the company that developed it; probable reasons for this failure are the notoriously uncompliant target patient population, some critical issues such as the view that the patient’s psyche is downgraded to the status of a digital dataset(5), or the fact that the need for a stick-on wearable receiver is dismissed as technologically unacceptable in a Bluetooth world. On 17 July 2020, Otsuka withdrew the application for market approval filed with the European Medicines Agency(6).
Another example of digital medicine is the Propeller technology, developed for patients with asthma or chronic obstructive pulmonary disease (COPD). In this case, the sensor is applied directly to the inhaler and automatically registers where, when and how often the drug is used. This information is then relayed to an app on a smartphone(8). Approved for marketing on 7 July 2020, Propeller is the first such sensor to be authorized in Europe.
Though bracketed by regulatory classification in the medical devices category, the R&D and delivery characteristics of DTx are comparable to those of drugs. Unlike PSP and digital medicines, DTx products are developedby controlled clinical investigation, are subject to a regulatory approval process for specific indications, and are reimbursable and prescribable. What differentiates DTx from a conventional drug is the active principle, which is no longer a chemical or biological entity, but comprises algorithms and software. Like conventional drugs, DTx products too have excipients, such as modules for patient gratification, reminders to take the DTx and complementary treatments, as well as modules for patient contact with the GP and with other patients treated for the same indication. Within the panorama of digital health technologies, DTx are an independent category of evidence-based products whose principal function is to deliver therapeutic interventions generated by software, so as to prevent, manage or treat a disorder or disease(9). DTx can incorporate additional functions, to enable integration with health platforms, assessment of patient compliance, and combination with sensors or wearable devices. In addition, personalized treatment pathways can be generated, thus optimizing management of the condition according to the individual patient’s exact characteristics and bringing so-called personalized medicine up to date in terms of digital technology(10).
DTx can generate therapeutic interventions both on a stand-alone basis and in combination with conventional drugs, integrating software- and algorithm-based technological innovation with drug treatment. Stand-alone DTx products work autonomously, being designed as interventions able to change a patient’s dysfunctional behaviours and, in this way, substituting drug treatment. An example of this stand-alone use can be found in psychotherapy of insomnia, depression or attention deficit hyperactivity disorder (ADHD) and schizophrenia, where the development of software simulating cognitive behavioural therapy (CBT) models or psychoeducationalmodels has improved patients’clinical outcomes, enabling their direct engagement in disease management(10). The combination of DTx with conventional drugs, known as augment or add-on treatment, can mean that the two work together to improve the associated drug treatment’s clinical efficacy, safety and patient compliance. On the other hand, combination or combo therapy means that the combination of drug and DTx is devised as such from the clinical development phase, which aims to demonstrate the combination’s superiority over use of the drug alone(11).
DTx are a new opportunity, mainly for the treatment of chronic diseaserelated to lifestyle and dysfunctional behaviours, where conventional therapy proves only partly effective. DTx can correct these dysfunctional behaviours, stimulating the patient’s involvement and active participation, and providing information and support in disease management. For this reason, DTx are particularly suited to deliver treatments that are normally provided in face-to-face encounters, such as CBT and clinical hypnotherapy.The indications for DTx approved to date are for mental disease, metabolic diseases and dependencies. Delivery of DTx by digital devices can offer a number of advantages, such as: (i) easier access to treatment, including during leisure time and not limited to face-to-face meetings with the psychotherapist; (ii) personalization of treatment, on the basis of outcomes and progress observed; (iii) consistent quality of DTx, by comparison with the extremely variable quality of face-to-face treatment with a psychotherapist.
3. Regulatory agencies and DTx
The first DTx product approved by the FDA in 2017 was reSET®, an app developed by the Pear Therapeutics company, followed in 2018 by reSET-O®. Both reSET and reSET-O are examples of prescription DTx, indicated respectively for treatment of substance use disorder and opioid dependence. These treatments are delivered by apps for mobile devices that use a form of CBT known as the community reinforcement approach (CRA), originally developed for alcohol and cocaine dependence.
In June 2020, the FDA approved EndeavourRx, manufactured by AkiliTherapeutics, the first game-based DTx device for attention enhancement in children with ADHD. EndeavourRx is indicated for patients from 8 to 12 years with ADHD, as part of a programme that can include clinical treatment, medication and educational programmes(12). EndeavourRx is based on Akili Selective Stimulus Management (SSMETM), a technology designed for targeted activation of specific neuronal circuits, and thus for treatment of diseases with associated cognitive dysfunctions.
SomrystTMis a DTx product approved in 2020 by the FDA for treatment of chronic insomnia, using CBT interventions (CBT-I) in line with the American Academy of Sleep Medicine guidelines as the first-line treatment for chronic insomnia(13). SomrystTMalso provides information on the progression of insomnia, thanks to monitoring of treatment by the physician and healthcare staff on a dashboard that collects data on sleep metrics, clinical parameters like Insomnia Severity Index (ISI) score, and assessments of the patient’s quality of life through the Patient Health Questionnaire 8 (PHQ-8).
OleenaTMis the first DTx product developed for oncological indications, approved by the FDA in 2019(14). It is a PDT mobile app, designed to help oncological patients manage their symptoms and to enable remote monitoring by the medical team. OleenaTMprovides personalized recommendations for symptom management, thanks to evidence-based algorithms. This therapy requires the patient’s cooperation with the healthcare provider, since the device is initially configured by the latter on the basis of the patient’s clinical status, after which the patient will log directly on OleenaTMany symptoms experienced in association with their oncological condition.
In the United Kingdom, before Brexit the National Institute for Health and Care Excellence (NICE) had already set up a working group under the guidance of the National Health Service, to help companies and regulatory boards identify what type of evidence is most relevant to the assessment of digital tools, thus meeting both the NHS’and the patients’needs with a view to accelerating regulatory processes(15). This initiative is now obviously no longer situated in a European Union context. To date, three DTx products have been recommended for use by NICE, but not yet approved: Deprexis, an interactive medical device with an online base to accompany treatment of patients with unipolar depression or depressive mood disorders(16); Space from Depression, another online programme for treatment of depression, to be used under a therapist’s supervision as an alternative to conventional CBT(17); and BDD-NET, an online programme for treatment of moderate to severe forms of body dysmorphic disorder(18).
To date no prescribed DTx product is authorized in most European countries. In Europe, the regulatory system for DTx is still immature and there are no specific regulations for their assessment, or to guarantee their safety and the integrity of the data collected. The European and US regulatory classification of DTx includes them under the heading of medical devices(19-21).
With a view to market approval, a regulatory framework for these therapies is needed, with appropriate distinguishing features from health and well-being apps. Since DTx differ from health and well-being apps in that they can determine a therapeutic effect, for a product to be part of this class evidence of its efficacy and safety must be provided before market approval. This raises the need to establish a regulatory framework for the various types of DTx, with a view to determining what clinical investigations, and how many, are required for purposes of market approval.
4. Critical issues
Despite their documented advantages, DTx present some critical issues. Since the technology involved is still in the development phase, it is not always easy to carry out clinical investigation able to generate sufficient evidence on safety and clinical efficacy. The digital endpoints used in clinicaltrials for DTx are different from those of traditional clinical trials, and their validation is made difficult by the absence of a gold standard. In addition, the technical characteristics of DTx could be rapidly updated during the course of the trial, and the technology itself could become obsolete even before the trial is over. These therapies must therefore be subjected to rigorous clinical investigation within a very limited time frame, to enable timely generation of robust scientific evidence(22).
The lack of specific regulations to guarantee the safety of these devices and the quality of the data collected is a further obstacle for the development of DTx. Post-marketing surveillance of efficacy and safety, particularly with regard to possible adverse effects like addictive potential or pseudo-specificity of the therapy itself, is therefore a fundamental activity that will have an increasingly important role with the growing uptake of DTx into clinical practice(3). More generally, the safety of these technologies is a particularly important consideration. In the STARS-ADHD clinical trial, 6.7% of subjects treated for ADHD with AKL-T01 (Endeavour) showed adverse events, in comparison with 1.8% of patients treated with an active control(23). Overall, adverse events recorded in studies on Endeavour involved 9.3% of patients. These included frustration, headache, emotional reactions, vertigo, nausea and aggressiveness(24).
In addition, digitalization of health and of the health system calls for greater patient education and engagement, promoting self-management and active involvement in the related decision-making; this process, however, has to be personalized and structured. Excessive patient empowerment could have a negative impact on the relationship with the physician: although these technologies can enable continuous dialogue between medical staff and the patient, there is the risk that unrestrained use of technology can lessen the need for a direct relationship with the specialist and, even more, with the GP.
Another critical issue associated with the use of these technologies, and specifically with wearable devices, is the management and sharing of patient data. Though the digitalization of healthcare is progressing slowly, the quantity of data that can be used for the patient has greatly increased in the last decade, making it necessary to guarantee patients’data protection and security with a view to supporting correct dissemination of DTx(25). In the United States, management of data obtained by medical devices is regulated by the Health Insurance Portability and Accountability Act, which makes patient consent mandatory for collection and sharing of data. However, data obtained by these devices can be shared in encrypted and aggregate form, without an explicit agreement as to who will have access to the data. In the European Union, the new General Data Protection Regulation (GDPR) does not distinguish between the various forms of digital device, but covers all data generated by wearable devices or health and well-being apps. In addition, the EU requires clearly defined aims for data use, together with patient consent for their re-use and dissemination; patient consent can be withdrawn at any time.
5. Future prospects
Given the growing digitalization of the health world, with an expected market turnover for DTx alone of about $9.4 billion by 2025(26), there is increasing interest in regulatory questions and R&D processes in relation to these technologies. The economic growth of the DTx sector could in future equal, or even exceed, that of conventional drugs, thus enabling greater access to patient-reported real-world data. In the last few years, a lot of pharmaceutical companies have started to cooperate with technology companies for development of DTx. This collaboration brings important opportunities for development and innovation. The increase in the quantity of data related to every feature of treatment and of the patient/user, along with the availability of increasingly refined statistical predictive systems, could conceivably usher in greater personalization of medical treatment and more thorough assessment of efficacy and safety, both for these treatments and for any drugs associated with them. It should also be noted that combined use of these treatments and of conventional drugs, with the same indication but a different mechanism of action, can determine summation or synergy in terms of efficacy and tolerability, thus bringing added therapeutic value.
The patient’s involvement in health-related decision-making processes and in the management of their own health must, however, not come at the expense of their direct relationship with the physician. A physician who fully appreciates the potential of these additional therapies will continue to play a fundamental role in correct assessment and clinical management of treatment, while admittedly leaving scope for greater independence of the patient. The prescriber will therefore have to educate patients to use these therapies correctly, ensuring not only that self-diagnosis and do-it-yourself treatments are out of the question, but also that more fragile patients do not find themselves having to cope with too great an emotional burden.
What is known
• Digital health offers new opportunities for addressing critical aspects of the health system and improving the quality of what is available, aiming at patient-centred treatment and its personalization
• There is increasing attention, among the medical and scientific community and regulatory authorities, to digitalization in the healthcare sector. This applies particularly to development of fully fledged DTx, and also of digital tools supporting conventional drug treatments, some of which have received regulatory approval and are now on the market
• Though DTx are placed for regulatory purposes in the medical devices category, their R&D and delivery characteristics are comparable to those of drugs. As such, they have to be developed by controlled clinical investigation and go through a regulatory approval process for specific indications, in order to be eligible for reimbursement and available for medical prescription
• DTx are a new therapeutic opportunity, mainly for treatment of some chronic diseases associated with dysfunctional lifestyles and behaviours in which drug treatment alone is only partially effective.
What is uncertain
• In regulatory terms, DTx are classed as medical devices; however, in Europe there is not yet a clearly defined regulatory procedure taking into account the specificities of these therapies, whose development requires more rapid lead times than conventional drugs
• The considerable quantity of sensitive data generated by these technologies raises a number of issues in terms of data use and patients’ data protection
• It is not yet clear how post-marketing surveillance of DTx safety can be set up, and which authority must be responsible.
What we recommend
• Any DTx must be integrated into a treatment programme, to be decided by the GP and shared with the patient
• As with traditional drugs, any DTx product’s placement on the market must be followed by close reassessment of its risk-benefit profile
• It is essential that there should be global harmonization of development and market approval procedures for DTx.
Bibliography
1.Pani L. Radical singularities and future of Pharmacology. Pharmadvances. 2019.
2.Innovazione digitale in sanità: i risultati 2019 [Internet]. Available from: https://www.impresasanita.it/it/articles/20190531/innovazione_digitale_in_sanita_i_risultati_2019.
3.Recchia G, Capuano M, Mistri N, Verna R. Digital Therapeutics-What they are, what they will be. Acta Sci Med Sci 2020; 4: 1-9.
4.Food and Drug Administration. FDA approves pill with sensor that digitally tracks if patients have ingested their medication [Internet]. 2017. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-pill-sensor-digitally-tracks-if-patients-have-ingested-their-medication.
5.Hatch AR. Digital mental health drug raises troubling questions [Internet]. 2018. Available from: https://www.phillyvoice.com/digital-mental-health-drug-cyborg-ethics-abilify-mycite/.
6.European Medicines Agency. Abilify MyCite: Withdrawal of the marketing authorisation application [Internet]. 2020. Available from: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/abilify-mycite.
7.Kryzhanovska A. 6 reasons To Build a Health Monitoring System for a Hospital [Internet]. 2020. Available from: https://gearheart.io/blog/6-reasons-build-health-monitoring-system-hospital/.
8.Propeller [Internet]. Available from: https://www.propellerhealth.com/.
9.Digital Therapeutic Alliance. Digital Therapeutics: Combining Technology and Evidence-based Medicine to Transform Personalized Patient Care. Available from: https://dtxalliance.org/wp-content/uploads/2018/09/DTA-Report_DTx-Industry-Foundations.pdf.
10.Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells T. Digital Therapeutics: An Integral Component of Digital Innovation in Drug Development. Clin Pharmacol Ther 2018; ; 104: 72-80.
11.S3 connected health. Digital Therapeutics: pharma’s threat or opportunity [Internet]. Available from: https://www.s3connectedhealth.com/resources/white-papers/digital-therapeutics-pharmas-threat-or-opportunity.
12.Food and Drug Administration. FDA Permits Marketing of First Game-Based Digital Therapeutic to Improve Attention Function in Children with ADHD [Internet]. 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-first-game-based-digital-therapeutic-improve-attention-function-children-adhd.
13.Schutte-Rodin SL, Broch L, Buysee D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4: 487-504.
14.Oleena [Internet]. Available from: https://oleena.com/.
15.National Institute for Health and Care Excellence (NICE). Evidence standards framework for digital health technologies: user guide [Internet]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/user-guide.pdf.
16.National Institute for Health and Care Excellence. Digital psychological therapy briefing – Deprexis for adults with depression [Internet]. 2018. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-advice/IAPT/iab-deprexis.pdf.
17.National Institute for Health and Care Excellence (NICE). IAPT assessment briefing – Space from Depression for adults with depression [Internet]. 2018. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-advice/IAPT/IAB-space-from-depression.pdf.
18.National Institute for Health and Care Excellence (NICE). BDD-NET for adults with body dysmorphic disorder [Internet]. 2019. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-advice/IAPT/iab-bdd-net-adults-body-dysmorphic-disorder-for-publication.pdf.
19.Attuazione della Direttiva 93/42/CEE concernente i dispositivi medici. Decreto lgs. 24 febbraio 1997, n. 46 emendato col D. lgs. 25.01.2010, n.37 – Recepimento Direttiva 2007/47/CE [Internet]. Available from: http://www.salute.gov.it/imgs/C_17_pagineAree_1636_listaFile_itemName_1_file.pdf.
20.Gazzetta Ufficiale dell’Unione Europea. Regolamento (UE) 2017/745 del Parlamento Europeo e del Consiglio [Internet]. Available from: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=CELEX%3A32017R0745.
21.Food and Drug Administration. Software as a Medical Device (SaMD) [Internet]. 2018. Available from: https://www.fda.gov/medical-devices/digital-health/software-medical-device-samd.
22.Chung J-Y. Digital therapeutics and clinical pharmacology. Transl Clin Pharmacol 2019; 27:6-11.
23.Kollins SH, DeLoss DJ, Cañadas E, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Health 2020; 2: E168-E178.
24.Endeavour TM. Instruction for use. Akili Interactive Labs. 2020.
25.Gopal G, Suter-Crazzolara C, Toldo L, Eberhardt W. Digital transformation in healthcare – Architectures of present and future information technologies. Clin Chem Lab Med 2019; 57: 328-35.
26.Grand View Research. Digital Therapeutics Market Growth & Trends [Internet]. 2017. Available from: https://www.grandviewresearch.com/press-release/global-digital-therapeutics-market.



