Giuseppe Recchia1,2, Paolo Barbanti3, Lorenzo Cottini4, 5, Antonio Ferrari6, Oriana Ciani7, Emanuele Lettieri8
1daVinci Digital Therapeutics srl, Milano
2Smith Kline Foundation, Verona
3Pharma & Biotech Advisors srl, Milano
4Association of Industrial Pharmacists (Associazione Farmaceutici Industria – AFI), Milano
5High Research srl, Milano
6IQVIA Ltd, Durham, NC, United States
7CERGAS, SDA Bocconi School of Management
8Department of Management Engineering, University “Politecnico” of Milano
Tendenze nuove, Numero Speciale 4 2021; 127-136: DOI: 10.32032/TENDENZENUOVENS04202108.PDF

Digital therapeutics, as a new medical intervention method and therapeutic option for the patient, can enhance outcomes of chronic diseases and dependencies(1). They also afford a novel opportunity, particularly relevantin light of the new normal resulting from CoViD-19, for development of Italy’s and Europe’s technological and economic ecosystem.
The development of digital therapeutics is ushering in an emerging health market that is constantly growing, with new actors and new rules (definition and discussion of which are still, to a certain extent, work in progress). The only viable competitors on this new market will be those based in countries that have created an environment conducive to development and innovation, in terms both of products and of organizational models(2).
Digital therapeutics and the market
Estimates of market size and trends in the next 10 years have been the subject of considerable investigation by specialized research groups, whose conclusions in most cases are fairly consistent.
The global market for digital therapeutics was worth $1.7 billion in 2019. According to current estimates, it could reach $9.4 billion by 2028, with a compound annual growth rate (CAGR) of 22.1% between 2020 and 2028(3). Other estimates indicate that the $9 billion threshold can be reached as early as 2025, and that use of digital therapeutics by patients will have grown more than tenfold by 2023(4).
Among the major global determinants of market growth for digital therapeutics are the increasing need to control health costs related to higher incidence and prevalence of chronic diseases, development of disease managementmodels in which the patient takes on a progressively more active role, the increase in missed diagnoses, and the reduction in lead time for a correct diagnosis. Further factors are the more widespread use of interactive devices (smartphones and tablets), the development of health(care) applications, and the growing number of partnerships or collaborations between pharmaceutical and technological companies. However, these growth factors are offset by other considerations that can slow down or hinder adoption of digital therapeutics and the related market growth – particularly in relation to provision for patients’ personal data protection, and the overarching issue of risks and threats to information security. Other limiting factors, particularly in Italy, are the low awareness of these new therapeutic tools and, more generally, the poor digital literacy of the population at large, as well as pushback from some providers of traditional health services.
Market segmentation
Segmentation of the global digital therapeutics market is mainly by therapeutic indication and distribution channel.
In terms of indications, the market breaks down as follows:
• metabolic diseases
• cardiovascular diseases
• central nervous system diseases
• respiratory diseases
• smoking cessation
• musculoskeletal diseases
• renal diseases
• other.
Metabolic diseases (particularly type 2 diabetes) are the segment with the potential to grow fastest and to achieve the greatest increase in the next five years. The growing prevalence of diabetes contributes to the increasing demand for digital therapeutics, and is one of the main development factors for this market.
In terms of distribution channel, the digital therapeutics market breaks down into the following segments:
• patients (B2C – Business to Consumer)
• health service providers (B2B – Business to Business)
• private sector payors, such as insurance companies (B2B – Business to Business)
• public sector payors, such as regional or national health services (B2G –Business to Government, or B2A – Business to Administration)
• employers (B2B – Business to Business).
B2B is currently the sector with the largest market share and the highest expected CAGR. This trend (particularly in the USA, which will continue to be the leading country in the development of digital therapeutics over the next few years) is related to growing uptake of digital therapeutics by health service providers and private payors, as well as by employers running employee benefit schemes.
In Europe, and particularly in countries with mainly state-financed health services such as Italy, increasing use of digital therapeutics and related market growth will be dependent on development of B2G models, enabling widespread access and reimbursement by public health services in the same way as for drug treatments(5).
Territorially, North America is currently the largest market in the world, thanks to growing investments in digital therapeutics, the large and ever-increasing number of start-ups in this field, and the government support that gives them a decisive boost(6).
The European digital therapeutics market was valued at $503.48 million in 2018, and is expected to reach $2.3 billion by 2026, with a CAGR of 20.6%(3).
Various surveys agree that emerging markets such as Brazil and India can offer market players significant growth opportunities(3,4).
International companies
The major players in this sector are mostly located in the USA, and to a far lesser extent in Europe and elsewhere. Some of the companies currently to the fore in digital therapeutics are listed in Table 1.
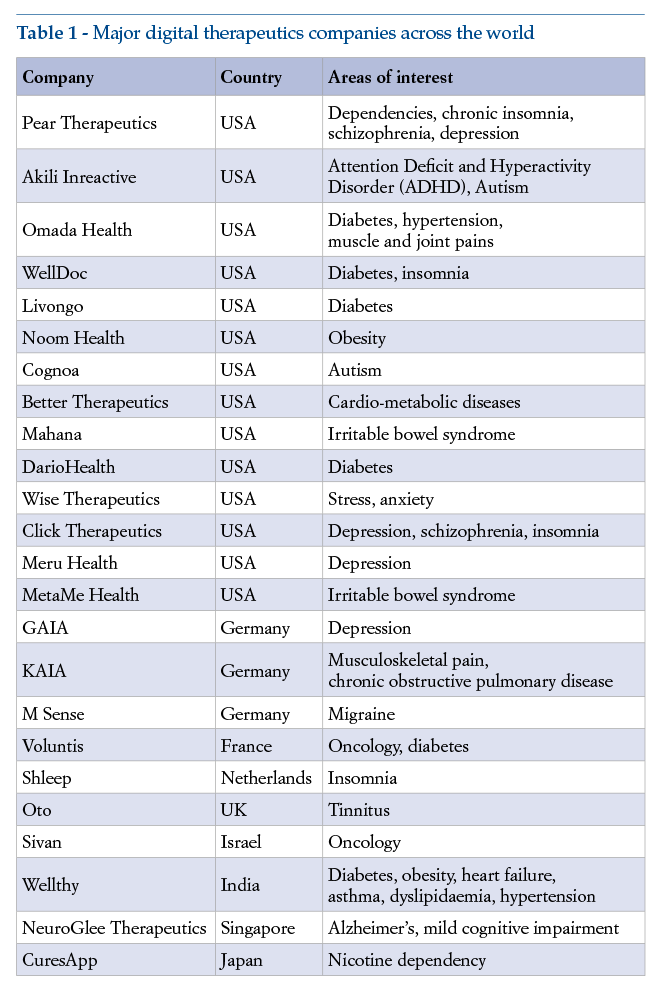
Most of the well established, larger digital therapeutics companies belong to the Digital Therapeutics Alliance (DTA), a non-profit associationfounded in 2017. The DTA comprises leading specialist companies and otherstakeholders committed to development of digital therapeutics, with an international focus on the digital therapeutics business, their head office in the USA and a section dedicated to Europe. The mission of the DTA is to disseminate knowledge and use of clinical research-based digital therapeutics, and their integration into care pathways, by means of training; and to broaden the understanding, adoption and integration of clinically evaluated digital therapeutics with patients, clinicians, payors and policymakers, through education, advocacy, and cross-industry collaboration, the ultimate aim being to enhance clinical and economic health outcomes(8).
Digital biotech companies in Italy
The primary actors in research, development and market placement of digital therapeutics in Italy are innovative start-ups, which intervene in different capacities at the various levels of the digital therapeutics development chain. These are companies that – in their own right or through strategic partnerships – integrate and organize the entire supply chain, from software design and development to implementation of the clinical programme and market placement. As such, they can be defined as digital biotech companies.
To fulfil this definition, the companies concerned must explicitly declare that they document the therapeutic value of their products on the basis of confirmatory evidence obtained from randomized, controlled clinical trials.
In other cases, the companies concerned are specialized in specific technological activities, such as the development of serious games, of virtual reality, or of digital excipients. In these cases, it is the collaboration with digital biotech companies, as defined in the previous paragraph, that qualifies the actors concerned for inclusion in the category of digital therapeutics companies.
Despite their limited size in quantitative terms, digital biotech companies work in close contact with the academic world and are primary centres for innovation and knowledge production, enabling dissemination of know-how for purposes such as education. By maintaining close contact with international research groups and companies, digital biotech companies contribute to the overall development of Italy’s advanced knowledge-based ecosystem.
However, this development is currently limited by the absence of clear regulatory guidelines regarding access to digital therapeutics, above all in terms of eligibility to be classified as such, reimbursement policy and the related conditions.
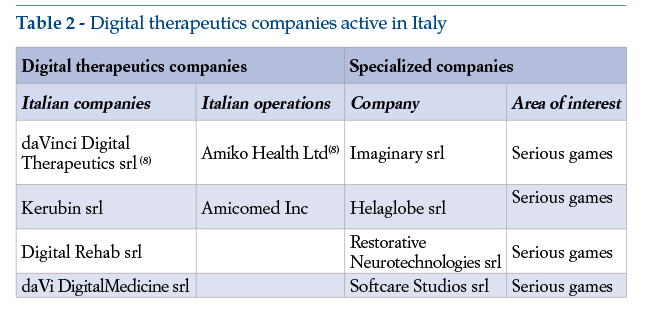
A survey carried out via LinkedIn, in October 2020, looked at Italiancompanies involved in various ways in digital therapeutics R&D and delivery. Table 2 shows the companies concerned, two of which are included in the list of 1000 International digital health companies(8).
Pharmaceutical companies and digital therapeutics start-ups
Digital therapeutics have been seen by some as a potential threat to pharmaceutical companies, for which the perceived danger is that they could find themselves relegated by digital technology companies into a position of disintermediation vis-à-vis the patient(9).
We take the opposite view and consider digital therapeutics as an important new opportunity for pharmaceutical companies, thanks above all to the added value they offer the patient on drug treatment(10). In this regard, collaboration towards development of new digital therapeutics productscan offer pharmaceutical companies a number of benefits:
• improvement of health outcomes for the patient
• enhanced value of the drug
• extension of the drug’s life cycle
• access to real-time/real-world information
• personalization of drug treatment
• completion of the therapeutic armamentarium
• entry into new therapeutic areas.
Use of digital therapeutics by the patient generates a considerable quantity of data, related both to the disease under treatment and to the therapies used (digital, pharmacological, other). These are real-world data, generated by interaction with the digital therapeutics algorithm, by completing a questionnaire or by providing information; real-time data can also be relayed from a sensor or device used by the patient. If authorized by the patient in accordance with current regulations, real-world/real-time data on the drug can be shared with the pharmaceutical company, which thus has immediate access to a far greater quantity of data than those generated during clinical development.
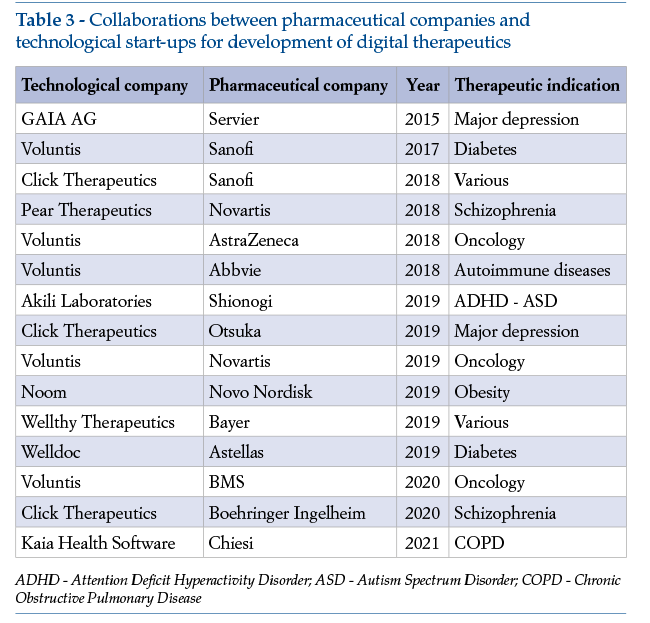
Given these opportunities, a number of pharmaceutical companies in the last few years have entered into collaborative agreements with technological companies for development of digital therapeutics (Table 3).
Digital therapeutics, Italy and Europe
To date, no digital therapeutics product is available in Italy and no Italiandigital biotech company has yet completed development of one in its own right, though the first digital therapeutics trials are now starting.
Digital therapeutics are nevertheless a major opportunity, both for Italy and for Europe. Italy, in particular, gives full expression to its excellence in the workroom, in the micro-company and in the small and medium enterprise, where R&D needs can be met by investments from within the Italian system. The entrepreneurial spirit and creativity that are the traditional strengths of the Italians thrive in small-scale initiatives, often in niche sectors. Examples are fashion, cars, motorcycles, shoes and other sectors where technology goes hand in hand with business flair, bearing witness to Italians’ aptitude and skills. All these features are present in the new digital therapeutics ecosystem, enabling Italy to act as a front runner in a way that would be hardly imaginable in other research sectors.
In order for Europe and Italy to be able to take on a leading role in this emerging area of digital technology and healthcare, it is first and foremost up to institutions, but also companies, investors, patients and representatives of civil society to align their interests, identifying digital biotechnology as a strategic area for the countries. It is only by joint effort that digital biotech and pharmaceutical companies will together be able to research, develop and sell new digital therapeutics products, and new combined drug digital therapies, on a worldwide market, therefore offering a better response to the patient’s expectations and contributing to the economic and social development of our continent. In this perspective, the fact the digitization as a whole has been considered one of the main missions of the post-CoViD-19 Recovery Plan outlined by the European Commission represents an important signal that must be adequately exploited.
What is known
• DTx are a new market
• The expected size of this market in 2028 is estimated at $9.4 billion, with a CAGR of 22.1% between 2020-2028
• The European DTx market is valued $503.48 in 2018 and is expected to reach $2.6 billion by 2026
• The DTx market in Italy is still non-existent
• Many companies (particularly based in the USA) are entering this market
• A number of DTx companies have been set up in the last few years in Europe and in Italy.
What is uncertain
• In the specific case of Italy, how can a DTx product become eligible for reimbursement?
• How can promotion of DTx be achieved in Europe and in Italy?
• How can a company ensure access and reimbursement for a combination between one of its drugs and a DTx product?
• How can European countries support international development of their DTx companies?
What we recommend
• In countries where this has not yet happened (for example Italy), define simple, clear rules for DTx, in relation to arrangements for access, reimbursement, scientific information and promotion
• Promote development of new European digital companies and sustain development of existing ones, by means of incentives, competitive bidding and dedicated initiatives
• Promote training programmes for doctors, both GPs and specialists, regarding digital medicine in general and DTx in particular
• Digitization is one of the main missions of the European Recovery Plan post-CoViD-19: efforts are needed to ensure that investments in this area are also significantly directed towards the life sciences.
Bibliography
1.Recchia G, Capuano DM, Mistri N, Verna R. Digital Therapeutics-What they are, what they will be. Acta Sci Med Sci 2020; 4: 134-42.
2.Borgonovi E, Filetti S, Lopane F. Condizioni organizzative abilitanti per le terapie digitali in Italia. Tendenze Nuove 2021; Speciale 1: 133-46.
3.Digital Therapeutics Explained: DTx Trends & Companies in 2020 – Business Insider.https://highsocietyinvestors.com/2020/09/19/the-digital-therapeutics-explainer-how-digital-treatments-could-be-a-9-billion-opportunity-by-2025-2.
4.Business Insider Intelligence The Digital Therapeutics 2019.
5.Martini N, Calabria S, Recchia G, et al. Terapie Digitali, HTA e Rimborso in Italia. Tendenze Nuove 2021; Speciale 1: 111-22.
6.Rodriguez JA, Clark CR, Bates DW. Digital Health Equity as a Necessity in the 21st Century Cures Act Era. JAMA 2020; 323: 2381-2. doi: 10.1001/jama.2020.7858.
7.Digital Therapeutics Alliance. https://dtxalliance.org.
8.Digital Health Market: Focus on Digital Therapeutics (2nd Edition), 2020-2030. Roots Analysis.https://www.rootsanalysis.com/reports/view_document/digital-health-market/208.html.
9.Digital Therapeutics: pharma’s threat or opportunity.https://www.s3connectedhealth.com/resources/white-papers/digital-therapeutics-pharmas-threat-or-opportunity.
10.daVinci Digital Therapeutics. Terapie Digitali e Farmaco, sostituzione o combinazione? https://terapiedigitali.davincidtx.com/terapie-digitali-e-farmaco/.



